ASIA ELECTRONICS INDUSTRYYOUR WINDOW TO SMART MANUFACTURING
Aqua Plasma Supports Reduction of Oxidized Copper
Samco Inc. focuses on product development with emphasis on environment, health, and safety (EHS). Moreover, the company has been advancing research on water vapor plasma at low pressure , Aqua Plasma®, and product development using the treatment technique. Thus far, the company has introduced reduction effects of oxidized copper and silver using Aqua Plasma®.
Conventional methods to reduce the oxidized copper include hydrogen reduction and hydrogen plasma reduction. It has been reported that these methods use atomic hydrogen (H) and proton (H+) for reduction. However, hydrogen has a wide range of explosion limits of 4 to 75 vol% in the air. Therefore, it needs to be handled with care and safety equipment.

Water vapor excels in terms of EHS with low inflammable property. However, thus far, it has been used as an oxidizing agent, such as thermal oxidation of silicon wafers.
Meanwhile, Samco presumed that water vapor would work as a reducing agent because it emits strong atomic H emission in plasma at low pressure.(1) As a result of the investigation, the company has found that water vapor can be used for the reduction of oxidized metal films as well. This article introduces the results of the latest research wherein the company studied the reduction mechanism of the oxidized copper using Aqua Plasma® and compared with hydrogen plasma.(2)
Experiment
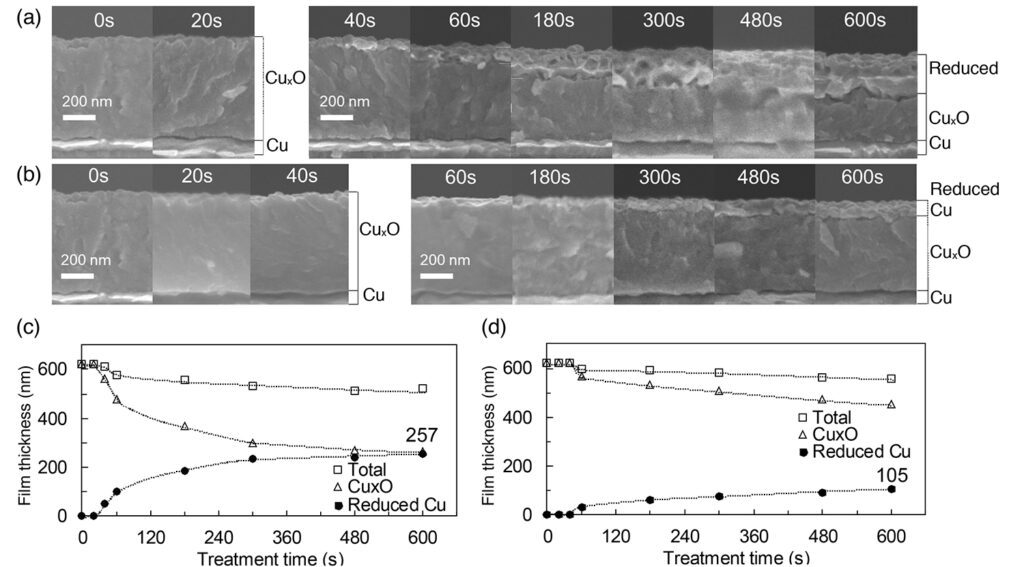
In this study, copper thin film which was deposited on a silicon wafer by electron beam deposition of copper with 99.99 percent purity was used as a sample. Then, the oxidized copper layer was formed by thermally processing on a hot plate at 250℃ for 30 minutes in the ambient air. As a result of X-ray diffraction and X-ray photoelectron spectroscopy (XPS)analyses, the oxidized copper sample was composed mainly of Cu2O with CuO existing only in the outermost surface (19nm in the surface layer at the deepest).The depth of oxidized copper layer was identified approximately 600 nm from the results of the depth direction analysis by the XPS and length measurement of the cross section of the cleaved sample using a scanning electron microscope (SEM).
The surface treatment was performed using Aqua Plasma® Cleaner (Photo 1). The sample was set to room-temperature ground electrodes and treated without applying heat. It was processed by fixing the water vapor flow rate, vacuum pressure and power, and changing treatment time. Hydrogen plasma was processed under the same conditions except for the gas (hydrogen with purity of 99.999%).
Results and Discussion
Fig. 1 shows the thickness of the reduced copper layer measured by SEM observation of the cross section and plotted by treatment time. In addition, the thickness of oxidized and total copper layer with the results of hydrogen plasma.
An induction period was observed in which the oxidized copper layer was not reduced until 40 or 60 seconds after the plasma ignition. The depth of the reduced copper layer increased not linearly but along an S-shape curve with respect to the treatment time. This tendency were consistent with characteristics reported in previous studies(3, 4) on hydrogen plasma.
The hydrogen plasma in this study also showed the similar tendency of that. These results indicate the oxidized copper in Aqua Plasma® can be reduced by H and H+ as in the case of hydrogen plasma. For further details of the results and discussion, please refer to reference (2).
In contrast, the depth of the reduction by Aqua Plasma® was 257 nm after 600 seconds, which was more than twice that of 105 nm by hydrogen plasma. Therefore, Aqua Plasma® enables to shorten the reduction-process time. Samco considers that adsorption of polar molecules with OH to the copper surface contributes to the difference in the reduction rate. The company intends to continue further investigation in this area.
References:
(1)H. Terai, R. Funahashi, T. Hashimoto, and M. Kakuta, “Heterogeneous bonding between cyclo-olefin polymer (COP) and glass-like substrate by newly developed water vapor-assisted plasma, Aqua Plasma Cleaner,” Electr. Eng. Japan, vol. 205, no. 4, pp. 48–56, 2018.
(2)H. Terai, K. Okafuji, T. Tanaka,T.Hashimoto, H. Nakano, and O. Tsuji,“Reduction of Copper Oxide by Water Vapor Plasma at Low Pressure,” IEEJ Trans. Sensors Micromachines, vol. 139, no. 7, pp. 157–162, 2019. (in Japanese)
(3)J. Y. Kim, J. A. Rodriguez, J. C.Hanson, A. I. Frenkel, and P. L. Lee, “Reduction of CuO and Cu2O with H2: H embedding and kinetic effects in the formation of suboxides,” J. Am. Chem. Soc., vol. 125, no. 35, pp. 10684–10692, 2003.
(4)K. C. Sabat, R. K. Paramguru, andB. K. Mishra, “Reduction of Copper Oxide by Low-Temperature Hydrogen Plasma,” Plasma Chem. Plasma Process., vol. 36, no. 4, pp. 1111–1124, 2016.
About This Article:
Samco, Inc. provided the contents of the article.




