ASIA ELECTRONICS INDUSTRYYOUR WINDOW TO SMART MANUFACTURING
Novel Production Test Yields Quality Medical Devices
The advent of IoT is driving transformation in healthcare. Rising healthcare costs and consumer demands for better healthcare delivery are driving widespread use of IoT in healthcare. This evolution is commonly known as the Internet of Medical Things (IoMT).
It is usual nowadays to see connected medical devices, such as ultrasound imaging systems, glucose monitors, bedside monitors, pacemakers, among others. With IoMT devices, non-critical patients can stay at home and healthcare professionals can monitor them through these connected devices. This decreases hospital admission rates and reduce costs.
Experts predict overall IoMT market to grow from US$72.5 billion in 2020 to US$188.2 billion in 2025 at compound annual growth rate (CAGR) of 21 percent1. This shows the influence of IOT in healthcare segment, from monitoring to diagnostic, to surgery and patient care.
Medical device manufacturers face unique challenges when incorporating IoT into medical devices, applications of which are mission critical. Therefore, they must be very dependable and long lasting.
The wireless connectivity needs always-on and dependable 24/7, all-year-round. It needs to work seamlessly in difficult physical environments.
These requirements are pressuring medical device manufacturers to implement dependable, efficient, and cost-effective manufacturing test strategies.
Importance of Effective Manufacturing Test
Design engineers always subject medical devices to thorough characterization during the design state to ensure quality. However, in manufacturing, assembly process variation, supply chain component deviation, test system repeatability, and operator overseeing errors can introduce failure into the device.
Manufacturing test may not be able to detect some of these defects because of lack of coverage in the test system. Marginally passed units may cause field failures during actual usage due to degraded performance.
To stay competitive in the market, there is a need to optimize manufacturing tests to achieve low cost-of-test but with fast test time to meet market cost expectation. Device testing must meet sufficient minimum conditions.
For instance, a wireless medical device OEM recently faced issues with the effectiveness of their manufacturing test setup. A custom version of the Bluetooth Low Energy (BLE) device managed to pass all manufacturing tests, only to show intermittent connection issues later. After troubleshooting, the device showed distorted antenna pattern, causing lower power in some of its BLE channels. In production testing, a quite simple connection test was not able to catch these intermittent connection issues during actual operation.
Capital Equipment Cost vs Potential Savings
A defect detected during initial manufacturing phases may not cost a lot to fix. However, the cost increases exponentially if the detection happens after production test or in the field during end user’s applications.
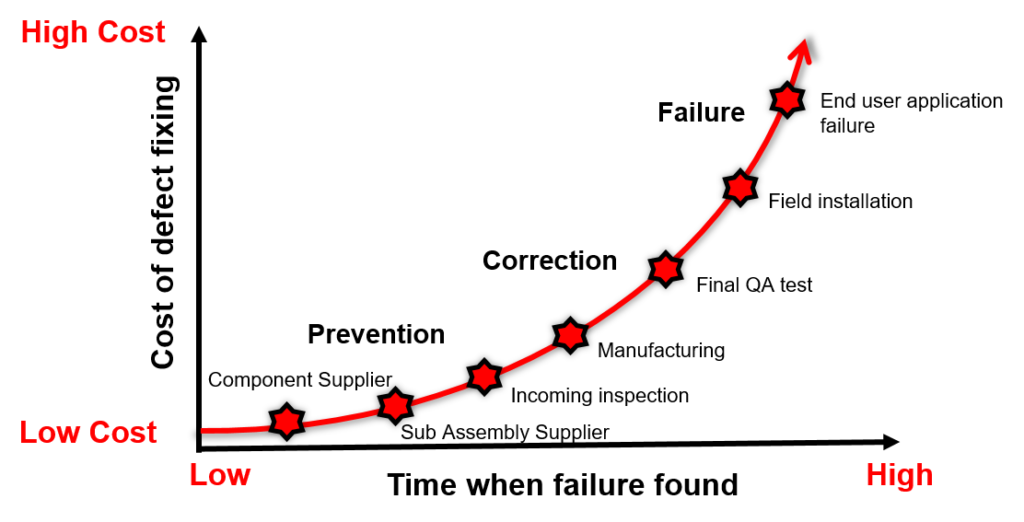
Investing in the right test solution is important during the new product introduction (NPI) phase. It may look expensive to invest in an RF tester and the required operators to execute the tests correctly. There are also annual maintenance and calibration costs.
However, the potential savings from detecting the failure early during manufacturing saves the direct and indirect or hidden costs of a field failure. The hidden cost due to warranties, failure troubleshooting, handling of replacement units, loss of sales due to bad reputation, or even penalties arising from use of defective products, can potentially total huge costs.
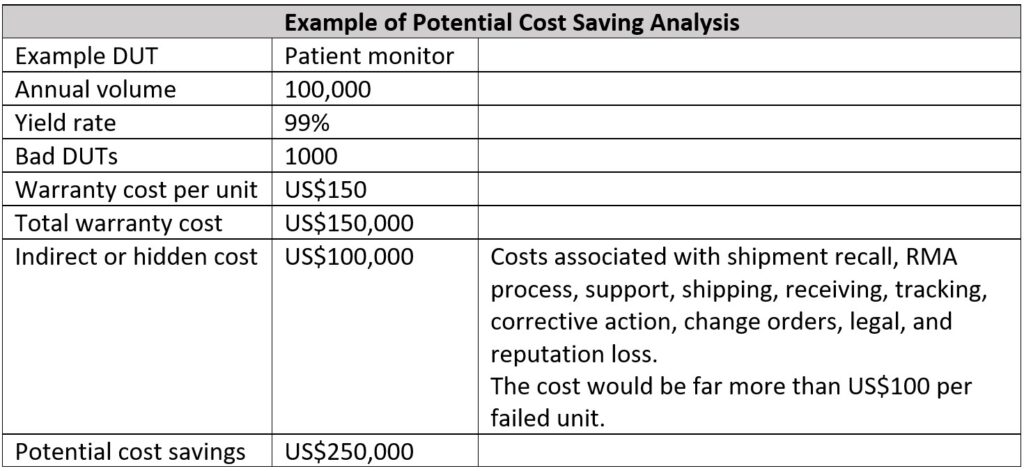
With the correct test strategies implemented in production, manufacturers can easily recoup the initial RF tester investment within the first year. The table below shows an example of potential cost savings. With the right test strategy, the potential cost saving can go up to US$250,000, with savings in warranty cost, and indirect/hidden cost in the event of shipment recall and so forth.
The next section discusses case studies of how leading medical device manufacturers are optimizing their manufacturing tests to ensure device quality, increase manufacturing yield, improve test system flexibility, or improve manufacturing throughput.
Ensures Quality of BLE Wireless Charger
It was the first attempt for a medical device company to incorporate wireless connectivity into their products. This company was developing a BLE enabled wireless charger. By enabling wireless connectivity to the charger, users can easily monitor the charging state and the battery level to prolong the battery life. During the design phase, the engineer needed to validate that the antenna and the matching circuits were performing according to the design goals. As the product developed makes use of an RF module, the engineer skipped the full parametric testing according to the Bluetooth requirements. The module maker guaranteed the RF performance.
As the engineer made modifications on the reference design and the antenna to fit into their form factor requirements, the engineer had to run full validation at the end device level to ensure the device was transmitting and receiving BLE signals properly in various end user scenarios. The company used an over-the-air (OTA) wireless tester specifically designed for IoT applications, to perform transmitter output power measurements and receiver packet error rate (PER) and sensitivity measurements.
The engineer used OTA measurements to validate the overall device transmitter and receiver performance including the antenna. The engineer could also choose to assess all 40 BLE frequency channels, or selectively test any channels of interest. With this capability, the engineer could validate the performance of the radio covering the entire BLE frequency band.
The manufacturer also uses the same test setup in manufacturing test, as it is cost-effective and simple enough for operator use. The optimized production test performed TX power and RX PER test at only three frequency channels – the lowest, middle, and highest frequency channels, to quickly validate the device performance over the entire BLE frequency band. This has helped the medical device manufacturer accelerate production and minimize correlation issues that frequently happen due to different test setups used in design and manufacturing.
In this case, the manufacturer has saved weeks of test development during the pilot phase, reduced time-to-market, and ensured device quality by adopting an effective test solution that offers the required test coverage.
Improves Yield for Wirelessly Controlled Surgical Machine
A manufacturer faced yield issues with their high-end surgical machine, which includes a wireless subsystem for remote control purposes. The wireless subsystem worked properly until failures started to show up. This became a big problem and impacted their shipments, as they only discovered failures after they completed the build and test of the machine. When the subsystem failed, they had to spend a long-time troubleshooting, repairing, and retesting. This caused inventory pileups and shipping errors. To fix this problem, they used a simple and cost effective IoT signaling tester to do a pre-screening test of the wireless modules before their installation into the wireless subsystem in their machine. Identifying defect modules before installation gave the manufacturer tremendous savings in test and repair time. This eventually allowed them to meet their daily output and yield targets.
Improves Production Flexibility for Low Volume Medical Wearables
A leading contract manufacturer manufactured medical wearables for many assorted brands. Their existing test solution was based on a non-signaling one-box-tester. Because the test conducted was in non-signaling mode, they loaded special test firmware to the device before testing. They removed and replaced with final production firmware after the test was complete. The maintenance of these large sets of firmware for various products were painful for the manufacturing team.
Operator overseeing error was also a key risk for them as they manufactured a wide range of devices from different customers. To improve the production flexibility and eliminate operator managing errors, they switched to an IoT wireless tester that enabled signaling OTA testing using final production firmware. This helped them to streamline the test processes and allowed them to easily switch between different product versions or brands.
The tester also supported major short-range formats like BLE 4.2, BLE 5, WLAN 2.4-GHz and 5-GHz so they could use the same test setup to test devices with different radio formats. Production flexibility is important for contract manufacturers to cater to volume fluctuations from their customers.
Increases Manufacturing Throughput, Reduces Cost
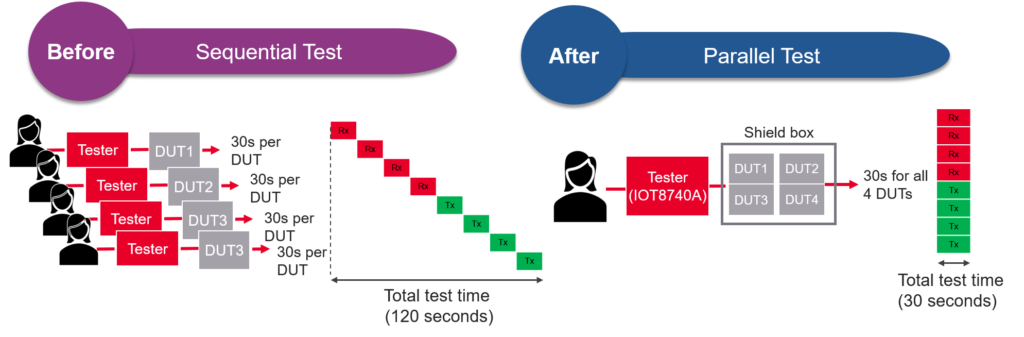
Part of the goal of a leading wearable manufacturer while investigating their next-generation test platform was to achieve higher throughput and reduce cost-of-test without sacrificing test coverage. They understood that quality is uncompromisable in the medical industry. Their existing test solution was time consuming and operator intensive. It involved manually putting the device-under-test (DUT) into the shield box, running the test, removing the DUT from the shield box once the tests completed, inserting a new unit, and repeating the process. It was running in sequential mode. Switching to a test solution that enabled parallel testing of multiple devices significantly reduced test time. The operator can put four devices into the same shield box at one time and run the required TX and RX tests simultaneously on all four devices. After the completion of tests, the operator removed all of them and replaced them with four new units to continue the test. With parallel testing capability, the manufacturer managed to cut test time by more than four times, resulting in significant throughput increases and cost of test reductions.
Conclusion
Connected medical devices are on the exponential growth trajectory. Adding wireless connectivity on medical devices brings tremendous conveniences to patients, allowing better healthcare delivery and lowering healthcare costs. The success of this megatrend will depend on the ability of medical device manufacturers to produce reliable and superior quality connected medical device that will not fail prematurely in the field. Effective manufacturing tests play a critical role in ensure the device quality by capturing defective or marginally passed devices that can potentially fail in the end user application. Selecting an effective test strategy will help to minimize this risk without incurring high manufacturing cost.

About This Article:
The author is Sook Hua, Industry Segment Manager with Keysight Technologies. She is the strategic solution planner responsible for Keysight’s Internet-of-things (IoT) solution portfolio expansion and marketing program planning to drive growth in the general electronic segment of Keysight Technologies.




